Master Sourcing High-Quality Ostomy Underwear for Global
Guide to Ostomy Underwear
- Introduction: Navigating the Global Market for ostomy underwear
- Understanding ostomy underwear Types and Variations
- Key Industrial Applications of ostomy underwear
- Strategic Material Selection Guide for ostomy underwear
- In-depth Look: Manufacturing Processes and Quality Assurance for ostomy underwear
- Comprehensive Cost and Pricing Analysis for ostomy underwear Sourcing
- Spotlight on Potential ostomy underwear Manufacturers and Suppliers
- Essential Technical Properties and Trade Terminology for ostomy underwear
- Navigating Market Dynamics, Sourcing Trends, and Sustainability in the ostomy underwear Sector
- Frequently Asked Questions (FAQs) for B2B Buyers of ostomy underwear
- Strategic Sourcing Conclusion and Outlook for ostomy underwear
Introduction: Navigating the Global Market for ostomy underwear
Unlocking Opportunities in the Global Ostomy Underwear Market
In the rapidly evolving landscape of ostomy care, the significance of specialized underwear designed for comfort, discretion, and skin health cannot be overstated. For B2B buyers across Africa, South America, the Middle East, and Europe—particularly countries like Turkey and South Africa—sourcing high-quality ostomy underwear presents a strategic opportunity to meet rising demand and improve patient outcomes. As the market expands, driven by demographic shifts, technological advancements, and increasing awareness, understanding the nuances of product types, materials, manufacturing standards, and supplier landscapes becomes essential.
This comprehensive guide is designed to empower international buyers with actionable insights to navigate this complex market confidently. It covers critical aspects such as different product categories—ranging from basic functional underwear to advanced, discreet solutions—alongside the latest material innovations that enhance comfort and durability. Additionally, it offers practical guidance on manufacturing quality control, evaluating suppliers, assessing costs, and understanding regional market dynamics influenced by regulatory and reimbursement policies.
By equipping you with in-depth knowledge and strategic considerations, this guide aims to facilitate smarter sourcing decisions, fostering partnerships that deliver value and reliability. Whether you’re expanding your product portfolio, entering new markets, or strengthening existing supply chains, this resource is your authoritative companion in capitalizing on the growing demand for ostomy underwear worldwide.
Understanding ostomy underwear Types and Variations
| Type Name | Key Distinguishing Features | Primary B2B Applications | Brief Pros & Cons for Buyers |
|---|---|---|---|
| Full-Body Ostomy Underwear | Designed as seamless, full-coverage garments with integrated pouching systems | Discreet, all-in-one solutions for post-surgical patients | Pros: High comfort, discreet appearance, reduces skin irritation; Cons: Higher manufacturing complexity, costlier, limited style options |
| Separatable/Modular Underwear | Features removable pouch modules attached to base underwear | Customizable solutions, suitable for varying patient needs | Pros: Flexibility, easier maintenance; Cons: Potential attachment issues, slightly bulkier, complex supply chain management |
| Disposable/Single-Use Underwear | Made from lightweight, breathable, often non-woven materials, intended for limited use | Short-term care, hospital settings, travel kits | Pros: Convenience, low maintenance; Cons: Environmental concerns, recurring procurement costs, less durable |
| Reusable/Washable Underwear | Crafted from durable fabrics with reinforced seams, designed for multiple washes | Long-term outpatient care, cost-sensitive markets | Pros: Cost-effective over time, eco-friendly; Cons: Requires proper laundering, potential for fabric degradation, higher initial investment |
| Specialty/Adaptive Underwear | Incorporates features like elastic waistbands, easy-open closures, or extra padding | Elderly, physically limited users, specific skin sensitivities | Pros: Enhanced accessibility, tailored fit; Cons: Niche market, potentially higher price point, limited standardized options |
Full-Body Ostomy Underwear
Full-body ostomy underwear integrates a seamless, form-fitting design with an embedded pouch system, offering maximum discretion and comfort. These are particularly suitable for post-surgical patients seeking an all-in-one, non-visible solution. B2B buyers should evaluate the manufacturing complexity, as seamless integration demands advanced textiles and assembly techniques, often resulting in higher costs. They are ideal for premium markets where patient comfort and aesthetic appeal are prioritized, such as Europe’s mature healthcare systems. Bulk procurement should consider supplier quality assurance and compliance with medical device regulations.
Separatable/Modular Underwear
This variation features a base garment with attachable or detachable pouch modules, providing customization according to patient needs. It allows for easier cleaning and replacement of the pouch without replacing the entire garment, making it suitable for long-term care facilities and outpatient services. Buyers should assess attachment mechanisms for durability and ease of use, as well as supply chain logistics for multiple components. Modular designs offer flexibility but may introduce complexity in inventory management and quality control, requiring reliable suppliers with consistent standards.
Disposable/Single-Use Underwear
Constructed from lightweight, breathable materials, disposable ostomy underwear is designed for short-term use, such as hospital stays or travel. These products appeal to cost-conscious markets and scenarios where convenience outweighs environmental concerns. B2B purchasers should weigh procurement costs against environmental impact and waste management infrastructure. Suppliers offering biodegradable or recyclable options are increasingly preferred, especially in regions with strict environmental regulations like Europe or South Africa. They are ideal for quick turnover needs but are less suitable for long-term or frequent use due to recurring costs.
Reusable/Washable Underwear
Durable, high-quality fabrics with reinforced seams characterize washable ostomy underwear, suitable for patients who prefer eco-friendly and cost-effective options. They are favored in markets emphasizing sustainability and long-term care, such as South America and parts of Europe. Buyers should consider fabric longevity, ease of laundering, and compliance with health standards. Initial investments are higher, but total cost of ownership decreases over time, making these products attractive for large-volume buyers and healthcare providers seeking environmentally responsible solutions.
Specialty/Adaptive Underwear
Designed with accessibility features like elastic waistbands, easy-open closures, or extra padding, specialty underwear addresses specific needs of elderly or physically limited users. These are essential in markets with aging populations, such as Turkey and South Africa, where ease of use and comfort are critical. B2B buyers should evaluate the availability of customization options, manufacturing lead times, and the potential for niche market growth. Although often higher priced, these products fulfill a vital role in inclusive healthcare, enabling broader patient access and adherence to treatment plans.
Related Video: One-Piece vs. Two-Piece Ostomy Systems: An In-Depth Look!
Key Industrial Applications of ostomy underwear
| Industry/Sector | Specific Application of ostomy underwear | Value/Benefit for the Business | Key Sourcing Considerations for this Application |
|---|---|---|---|
| Healthcare & Medical Devices | Post-operative patient support and incontinence management | Enhances patient comfort, reduces skin irritation, and improves compliance with medical protocols | Biocompatibility, hypoallergenic materials, durability, and adherence to medical standards (ISO, FDA) |
| Personal Care & Hygiene | Discreet, reusable underwear for ostomy and incontinence patients | Provides discreet, comfortable, and reusable options, reducing stigma and increasing patient satisfaction | Soft, breathable fabrics with moisture-wicking properties; ease of laundering; compliance with health regulations |
| Military & Emergency Response | Durable, quick-donning ostomy support wear for field use | Ensures hygiene and comfort in challenging environments, supporting operational readiness | Rugged, moisture-resistant materials; ease of application; high durability and washability |
| Sports & Active Lifestyle | Specialized ostomy underwear for athletes and active individuals | Combines discretion with support, enabling active lifestyles without compromising hygiene or comfort | Stretchable, breathable, moisture-wicking fabrics; secure fit; odor control features |
| Industrial & Occupational Health | Workers in hazardous environments requiring hygiene support | Maintains hygiene, prevents skin infections, and supports worker health in high-risk settings | Robust, protective, and easy-to-clean fabrics; compliance with occupational safety standards |
Healthcare & Medical Devices
Ostomy underwear in healthcare primarily supports post-operative recovery and incontinence management. These garments are designed to be worn directly against the skin, providing a discreet barrier that prevents leaks and skin irritation. International B2B buyers from regions like Africa, South America, and the Middle East should prioritize sourcing materials that meet strict biocompatibility and hypoallergenic standards, ensuring safety and comfort. Durable, skin-friendly fabrics that adhere to medical regulations help reduce complications and improve patient outcomes, making these products essential for hospitals, clinics, and home healthcare providers seeking reliable supply chains.
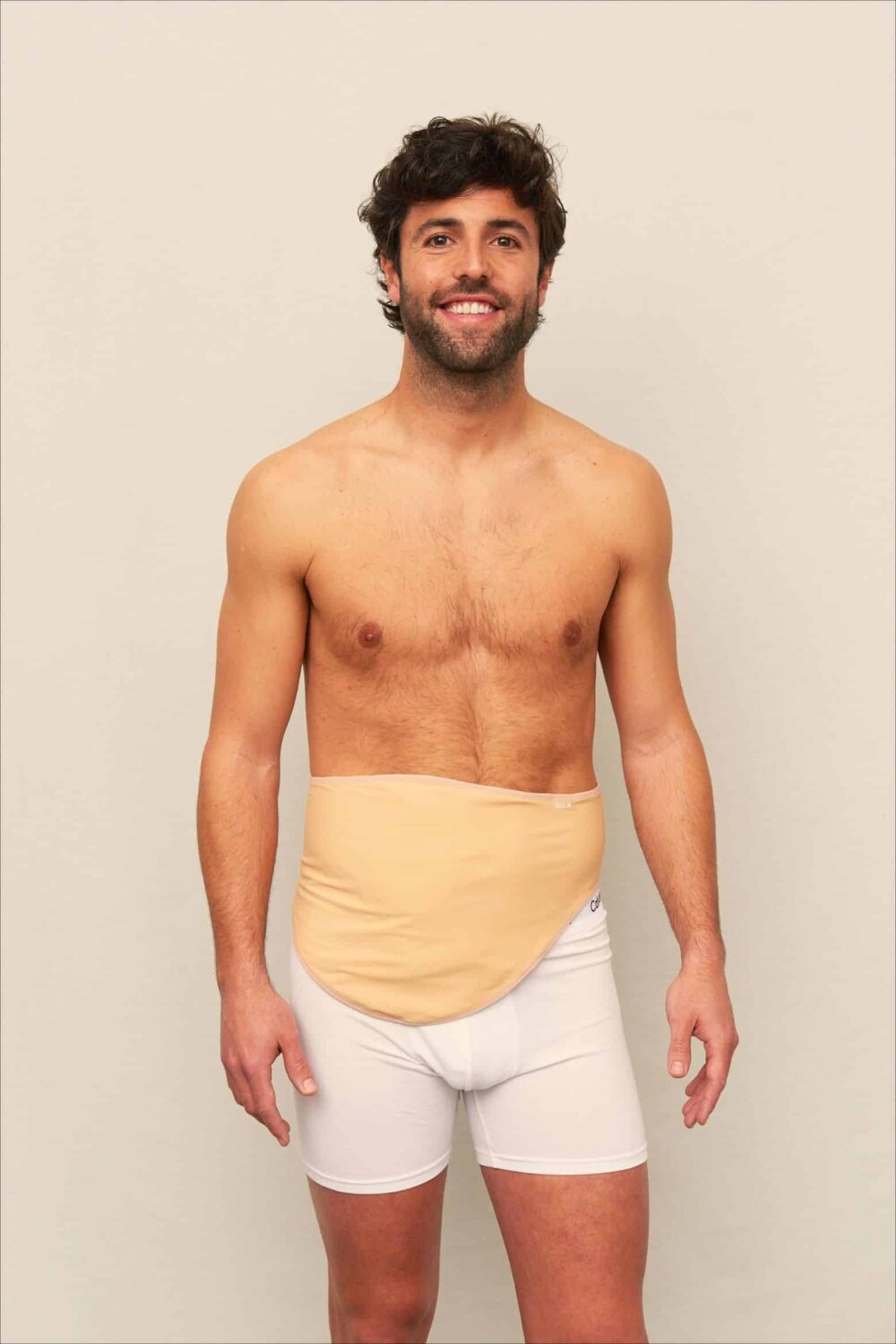
Personal Care & Hygiene
In the personal care sector, ostomy underwear offers a discreet, reusable alternative for individuals managing ostomy or incontinence issues. For buyers in Europe and emerging markets, emphasis should be placed on fabrics that are soft, breathable, and moisture-wicking to enhance comfort and reduce skin issues. Reusable designs that withstand frequent washing appeal to cost-conscious consumers and support sustainable practices. Sourcing high-quality, skin-friendly textiles that meet health and safety standards is crucial, especially for importers aiming to cater to markets with increasing awareness around hygiene and dignity.
Illustrative Image (Source: Google Search)
Military & Emergency Response
Durability and ease of use are critical in military and emergency response applications. Ostomy underwear designed for these sectors must withstand extreme conditions, including moisture, dirt, and rough handling. International buyers from regions like the Middle East and Africa should seek products with rugged, moisture-resistant fabrics that are quick to don and doff, ensuring hygiene and comfort in the field. High durability, washability, and material resilience are vital to maintain operational readiness and hygiene standards, especially in remote or resource-limited environments.
Sports & Active Lifestyle
Athletes and active individuals requiring ostomy support demand specialized underwear that balances discretion with performance. These products need to incorporate stretchable, breathable, and odor-control fabrics that allow freedom of movement while maintaining hygiene. For European and Asian markets, sourcing textiles with advanced moisture-wicking and antimicrobial properties ensures product appeal. International B2B buyers should focus on consistent quality, secure fit, and innovative fabric technologies to meet the demands of consumers seeking active lifestyles without compromising comfort or privacy.
Industrial & Occupational Health
In hazardous work environments, ostomy underwear can play a role in maintaining hygiene and preventing skin infections among workers. These garments must combine protective features with ease of cleaning and durability. Buyers in regions like South Africa, Turkey, and Latin America should prioritize sourcing fabrics that are high-resistance to chemicals, heat, and dirt, while also complying with occupational safety standards. Reliable, easy-to-maintain products support worker health, reduce downtime, and foster compliance with health and safety regulations in industrial settings.
Related Video: ConvaTec Ostomy Application Video
Strategic Material Selection Guide for ostomy underwear
Material Analysis for Ostomy Underwear
Selecting appropriate materials for ostomy underwear is critical for ensuring product performance, durability, comfort, and compliance with international standards. The choice of materials impacts not only the functional aspects, such as moisture management, skin compatibility, and barrier protection, but also influences manufacturing complexity and cost. Below is an in-depth analysis of four common materials used in this niche, tailored for international B2B buyers from Africa, South America, the Middle East, and Europe.
1. Polyester (PET) and Polypropylene (PP)
Key Properties:
Polyester and polypropylene are synthetic polymers widely used in medical textiles due to their lightweight, flexibility, and moisture-wicking capabilities. They offer good tensile strength, resistance to chemicals, and can be engineered for breathability or waterproofing. Polypropylene, in particular, is inherently hydrophobic, making it excellent for moisture barriers.
Pros & Cons:
– Pros: Cost-effective, easily available, high durability, and excellent chemical resistance. Polyester can be treated for enhanced breathability and moisture management. Polypropylene’s hydrophobic nature makes it suitable for barrier layers.
– Cons: Limited elasticity unless blended with elastomers, potential skin irritation if not properly processed, and environmental concerns related to microplastic shedding. Manufacturing requires precise control to ensure uniformity and biocompatibility.
Impact on Application:
Polyester and polypropylene are suitable for outer layers or barrier films in ostomy underwear, providing structural integrity and moisture resistance. They can be combined with other materials to improve comfort and skin contact quality.
International Considerations:
– Compliance: Must meet standards like ASTM F2100 (medical textiles), ISO 10993 (biocompatibility), and local regulations.
– Preferences: European markets favor recycled or bio-based PET to meet sustainability goals. Middle Eastern and African buyers often prioritize cost and availability, with growing interest in eco-friendly options.
– Manufacturing: Readily available globally, but quality control is essential to avoid variability in performance.
2. Nylon (Polyamide)
Key Properties:
Nylon is a synthetic fiber known for its excellent strength, elasticity, and abrasion resistance. It offers good moisture-wicking properties and can be engineered for softness and flexibility. Nylon’s chemical resistance makes it suitable for contact with bodily fluids and cleaning agents.
Pros & Cons:
– Pros: High durability, excellent elasticity, and good skin compatibility when processed appropriately. It can be made breathable with specialized coatings.
– Cons: Higher cost compared to polyester and polypropylene, susceptible to degradation under prolonged UV exposure, and may require additional treatments to enhance moisture management and antimicrobial properties.
Impact on Application:
Nylon is ideal for inner linings or comfort layers in ostomy underwear, providing a soft, flexible interface that reduces skin irritation. Its strength supports repeated washing and wear.
International Considerations:
– Compliance: Must adhere to standards like OEKO-TEX, ISO 10993, and regional textile regulations.
– Preferences: European and South American markets favor high-performance, eco-friendly nylon variants. Middle Eastern buyers may prefer cost-effective options with proven durability.
– Manufacturing: Widely available, but quality varies; sourcing from reputable suppliers ensures compliance and performance.
3. Spandex (Elastane or Lycra)
Key Properties:
Spandex is a highly elastic synthetic fiber that provides stretch and recovery, essential for snug-fitting ostomy underwear. It maintains shape over multiple wash cycles and enhances wearer comfort.
Pros & Cons:
– Pros: Exceptional elasticity, lightweight, and easy to incorporate into blends for stretchability. It improves fit and reduces skin chafing.
– Cons: Sensitive to heat and certain chemicals, which can degrade its elasticity over time. Cost is higher than basic fibers, and it requires careful handling during manufacturing.
Impact on Application:
Spandex is typically blended with polyester or nylon to produce a fabric that combines strength, stretch, and comfort. It’s vital for ensuring a secure fit around the stoma and peristomal area.
International Considerations:
– Compliance: Must meet standards like OEKO-TEX and regional chemical safety regulations.
– Preferences: European markets favor low-allergen, sustainably produced spandex. African and Middle Eastern markets prioritize cost-effective blends but are increasingly adopting high-performance variants.
– Manufacturing: Readily available globally, with many suppliers offering compliant, high-quality grades.
4. Bio-based and Recycled Polymers (e.g., Bio-PE, Recycled PET)
Key Properties:
Bio-based and recycled polymers are gaining prominence due to sustainability demands. These materials can mimic traditional plastics like PE and PET but with a lower environmental footprint. They often feature comparable barrier and mechanical properties.
Pros & Cons:
– Pros: Environmentally friendly, often compliant with strict regulations, and appealing to eco-conscious consumers. They support circular economy initiatives and can meet sustainability standards like GRS or FSC.
– Cons: Higher initial costs, limited availability in some regions, and potential variability in performance depending on the recycling process.
Impact on Application:
Suitable for barrier films and outer layers in ostomy underwear, especially where environmental compliance and branding are priorities. They can also enhance product appeal in markets with strong sustainability policies.
International Considerations:
– Compliance: Must meet regional standards such as EU Green Deal, REACH, and local regulations.
– Preferences: European and Middle Eastern markets are leading adopters, driven by regulations and consumer demand. African and South American markets show increasing interest but face supply chain limitations.
– Manufacturing: Growing availability, but buyers should verify certifications and material traceability.
Summary Table
| Material | Typical Use Case for ostomy underwear | Key Advantage | Key Disadvantage/Limitation | Relative Cost (Low/Med/High) |
|---|---|---|---|---|
| Polyester (PET) / Polypropylene (PP) | Outer barrier layers, moisture-resistant films | Cost-effective, high durability, chemical resistance | Limited elasticity, environmental concerns | Low |
| Nylon (Polyamide) | Inner linings, comfort layers | High strength, elasticity, skin compatibility | Higher cost, UV sensitivity | Med |
| Spandex (Elastane) | Stretch panels, snug fit zones | Excellent elasticity, shape retention | Sensitive to heat/chemicals, higher cost | Med |
| Bio-based/Recycled Polymers | Barrier films, eco-friendly outer layers |
In-depth Look: Manufacturing Processes and Quality Assurance for ostomy underwear
Manufacturing Processes for Ostomy Underwear
The production of ostomy underwear involves a complex integration of advanced textile engineering, medical-grade materials, and precise assembly techniques to ensure safety, comfort, and functionality. The process can be broadly divided into four main stages: material preparation, forming, assembly, and finishing.
Material Preparation
At this initial stage, raw materials such as medical-grade fabrics, superabsorbent polymers, waterproof membranes, and hypoallergenic adhesives are sourced. Suppliers must ensure these materials meet stringent biocompatibility and toxicity standards like ISO 10993. Material inspection includes verifying chemical composition, tensile strength, and permeability. For international buyers, verifying supplier certifications (e.g., ISO 9001, OEKO-TEX) is crucial to ensure consistent quality.
Forming and Fabrication
The fabric layers are cut using computer-aided design (CAD) and computer-aided manufacturing (CAM) systems to ensure precision and minimal waste. Advanced cutting techniques such as laser or die-cutting are employed to produce components with high accuracy. These layers typically include a moisture-wicking inner fabric, a waterproof barrier, and an outer textile layer that provides discretion and durability. For added comfort, some manufacturers incorporate breathable or antimicrobial treatments during this phase.
Assembly and Integration
The assembly process involves bonding or sewing the fabric layers together, often using ultrasonic welding or heat sealing for waterproof seams. Adhesives are carefully applied to attach absorbent pads or pouches, ensuring secure placement without compromising flexibility. The incorporation of elastic waistbands, fasteners, or adjustable features is also performed at this stage, with an emphasis on durability and comfort. The entire process is often conducted in controlled environments to prevent contamination, especially in medical-grade production facilities.
Finishing and Quality Checks
Final products undergo trimming, labeling, and packaging. Additional surface treatments like softening or anti-odor coatings are applied if required. Packaging is designed to preserve the integrity of the product during transit. The finished underwear is then subjected to rigorous quality control (QC) procedures before release.
Quality Control (QC) in Manufacturing of Ostomy Underwear
Quality assurance is paramount in the production of ostomy underwear due to the critical need for safety, reliability, and comfort. International standards and industry-specific regulations guide QC processes, which are implemented at multiple checkpoints throughout manufacturing.
International Standards and Industry Regulations
– ISO 9001: Provides a comprehensive framework for quality management systems, emphasizing process consistency, continuous improvement, and customer satisfaction. Most reputable manufacturers seek ISO 9001 certification to demonstrate their commitment to quality.
– ISO 13485: Specifically relevant for medical devices, including ostomy products, ensuring that manufacturers meet rigorous design, production, and traceability requirements.
– CE Marking (EU): Indicates compliance with European health, safety, and environmental protection legislation, including specific directives for medical devices (MDR 2017/745).
– FDA Regulations (U.S.): Require biocompatibility testing, sterilization validation, and proper labeling under 21 CFR Part 820 (Quality System Regulation).
QC Checkpoints and Testing Methods
– Incoming Quality Control (IQC): Raw materials are inspected upon receipt. Tests include chemical analysis, tensile strength, permeability, and biocompatibility assessments. Suppliers must provide Certificates of Analysis (CoA) to verify compliance.
– In-Process Quality Control (IPQC): During fabrication, parameters such as seam integrity, adhesive bonding strength, and dimensional accuracy are monitored. Non-destructive testing methods like ultrasonic testing are employed to detect seam leaks or weak bonds.
– Final Quality Control (FQC): The finished product undergoes comprehensive testing, including:
– Leakage Tests: Simulate real-use conditions to ensure waterproof barriers are intact.
– Adhesion and Bonding Tests: Verify the durability of bonded layers under stress.
– Skin Compatibility Tests: Confirm biocompatibility and hypoallergenic properties.
– Mechanical Durability: Assess stretchability, tear resistance, and elastic retention.
– Discreetness and Noise: Evaluate the product’s discretion and operational noise levels, critical for user comfort.
Certifications and Documentation
Manufacturers should provide detailed test reports, material certificates, and compliance documentation. For international buyers, especially from regions with specific regulations like Europe or South America, verifying the authenticity and validity of these documents is vital. Engaging third-party inspection agencies or certification bodies can ensure unbiased validation.
Supplier Verification and Audits
B2B buyers can verify supplier QC through:
– On-site Audits: Conducted by the buyer or third-party auditors to assess manufacturing practices, cleanliness, and process controls.
– Review of Quality Manuals and SOPs: Ensures that documented procedures align with international standards.
– Sample Testing: Independent laboratory testing of sample batches to verify compliance with technical specifications.
– Certifications Review: Confirm current validity of ISO, CE, FDA, or other relevant certifications.
Considerations for International B2B Buyers
For buyers from Africa, South America, the Middle East, and Europe, understanding regional regulatory nuances and supply chain robustness is essential. Manufacturers with ISO 9001 and ISO 13485 certifications are generally more reliable, but regional compliance must also be confirmed—such as CE marking for Europe or FDA approval for the U.S.
Buyers should prioritize suppliers who:
– Maintain transparent documentation and traceability of materials and processes.
– Participate in regular third-party audits and inspections.
– Offer comprehensive testing reports aligned with international standards.
– Demonstrate consistent product quality through samples or pilot runs before large-scale orders.
Engaging with experienced quality consultants or certification bodies can further mitigate risks, especially when entering new markets or working with suppliers unfamiliar with regional regulations. Building long-term partnerships with manufacturers committed to continuous improvement and compliance ensures sustained product quality and customer satisfaction across diverse international markets.
Related Video: Complete Process of Textile Manufacturing Fiber to Complete Garments
Comprehensive Cost and Pricing Analysis for ostomy underwear Sourcing
Cost Structure Breakdown for Ostomy Underwear
Understanding the comprehensive cost components involved in sourcing ostomy underwear is vital for international B2B buyers aiming for optimal pricing and quality. The primary cost drivers include:
-
Materials: High-quality, skin-friendly fabrics such as soft, breathable textiles, moisture-wicking layers, and elastic components are essential. Advanced features like odor control, discreet design, and antimicrobial treatments add to material costs, typically ranging from 20% to 35% of the total product cost.
-
Labor: Manufacturing labor costs vary significantly by region. For example, Turkish and South African factories may offer competitive rates ($1.50–$3.50 per piece), whereas Asian producers like China or India might offer lower wages ($0.80–$2.00 per piece). Skilled labor for quality control and assembly directly impacts product consistency and price.
-
Manufacturing Overhead: This encompasses factory utilities, machinery maintenance, and administrative expenses. Higher automation levels can reduce overhead costs but require initial capital investment, influencing overall pricing.
-
Tooling and Mold Costs: For customized designs or sizes, tooling expenses can range from $2,000 to $10,000 upfront. These costs are amortized over volume, making larger order quantities more cost-effective.
-
Quality Control & Certifications: Ensuring compliance with standards such as ISO, OEKO-TEX, or CE increases upfront costs but is crucial for market acceptance, especially in Europe and North America. QC costs might add approximately 5–10% to unit costs.
-
Logistics & Incoterms: Shipping costs depend on order volume, destination, and chosen Incoterms (e.g., FOB, CIF). For example, freight from China to Africa or South America can range from $0.50–$1.50 per unit in bulk, while faster air freight significantly increases costs.
-
Margins: Manufacturers typically apply a markup of 10–25% depending on the competitiveness of the market, order size, and relationship strength.
Price Influencers & Negotiation Levers
Several factors impact the final price you pay:
-
Order Volume & MOQ: Larger quantities (e.g., 10,000+ units) often secure better unit prices due to economies of scale. Many suppliers offer discounts for bulk orders, sometimes reducing unit costs by 15–30%.
-
Customization & Specifications: Standard designs are cheaper; custom features like specific colorways, branding, or unique fabric blends increase costs. Clear specifications upfront help prevent unexpected charges.
-
Materials & Certifications: Premium materials or eco-friendly options (recycled fabrics, organic cotton) carry higher costs but can command premium pricing and better market positioning. Certification costs should be factored into initial negotiations.
-
Supplier Factors: Established manufacturers with strong quality assurance systems and reliable supply chains may charge higher prices but reduce risks of delays or defects. Conversely, emerging suppliers might offer lower prices but require rigorous vetting.
-
Incoterms & Logistics: FOB (Free on Board) terms give buyers control over freight costs, while CIF (Cost, Insurance, Freight) includes logistics in the price, simplifying procurement but potentially raising initial costs.
Buyer Tips for Cost-Effective Sourcing
-
Negotiate for Volume Discounts: Emphasize long-term partnerships and bulk orders to leverage better pricing. Be transparent about your forecasted volumes.
-
Focus on Total Cost of Ownership: Consider hidden costs such as customs duties, import taxes, and potential quality-related rework. European and North American buyers should factor in compliance costs for certifications.
-
Leverage Multiple Quotes: Obtain quotes from suppliers in different regions—Turkey, South Africa, China, India—to compare prices and terms. Regional trade agreements (e.g., African Continental Free Trade Area, Mercosur) can reduce tariffs.
-
Evaluate Lead Times & Flexibility: Faster delivery may come at a premium. Balance your inventory needs with cost considerations to avoid excess stock or stockouts.
-
Build Strong Relationships: Trust and communication often lead to better payment terms, priority production, and shared innovations, ultimately lowering costs.
Indicative Price Range
While prices vary based on specifications and order size, typical unit costs for standard ostomy underwear range from $3.50 to $8.00 in large volumes. Premium, customized, or certified products may range from $8.00 to $15.00 per piece. These figures are approximate and should be validated through direct supplier quotes.
By carefully analyzing these cost components and influencing factors, international B2B buyers from Africa, South America, the Middle East, and Europe can optimize their procurement strategies, ensuring high-quality products at competitive prices while maintaining flexibility in negotiations and logistics planning.
Spotlight on Potential ostomy underwear Manufacturers and Suppliers
This section offers a look at a few manufacturers active in the ‘ostomy underwear’ market. This is a representative sample for illustrative purposes; B2B buyers must conduct their own extensive due diligence before any engagement. Information is synthesized from public sources and general industry knowledge.
Essential Technical Properties and Trade Terminology for ostomy underwear
Critical Technical Properties for Ostomy Underwear
1. Material Grade and Composition
The fabric and barrier materials used in ostomy underwear must meet stringent medical-grade standards, ensuring biocompatibility and durability. High-quality textiles such as moisture-wicking, breathable, and hypoallergenic fabrics are essential to enhance wearer comfort and minimize skin irritation. For the barrier layer, materials like hydrocolloid or silicone must conform to industry certifications (e.g., ISO 10993) to guarantee safety. B2B buyers should prioritize suppliers offering materials with consistent quality and compliance with relevant international standards to reduce product recalls and liability risks.
2. Tolerance and Dimensional Accuracy
Manufacturing tolerances refer to the allowable deviation in dimensions such as waistband size, pouch fit, and elastic properties. Precise tolerance levels (e.g., ±1 mm) ensure that the underwear fits securely across diverse body types and maintains functionality over repeated use. Tight control over tolerances reduces product returns and enhances customer satisfaction, which is vital for building brand reputation in international markets.
3. Barrier and Adhesive Performance
The barrier film’s permeability, leak resistance, and adhesion strength directly impact product efficacy. High-performance films must provide a reliable seal to prevent leaks while remaining discreet and comfortable. Adhesives should adhere securely to skin without causing irritation or damage during removal. B2B buyers should verify supplier test reports on barrier integrity and adhesive strength, ensuring compliance with medical device regulations and customer expectations.
4. Breathability and Discretion
Advanced fabrics incorporate micro-perforations or breathable membranes that allow airflow, reducing moisture buildup and skin maceration. This property is crucial for patient comfort and skin health, especially in humid climates. Discretion is enhanced through ultra-thin, low-profile designs that minimize noise and bulk, aligning with consumer demands for discreet, everyday wear.
5. Reusability and Washability
Some ostomy underwear are designed for multiple uses, requiring durable materials that withstand frequent washing without degradation of barrier properties or elasticity. B2B buyers should seek products with clear specifications on laundering instructions, longevity, and resistance to common detergents to ensure long-term value for end-users.
6. Regulatory Compliance and Certifications
All materials and manufacturing processes must adhere to regional standards such as FDA (US), CE (Europe), or PMDA (Japan). Suppliers should provide relevant certifications, test reports, and documentation demonstrating product safety, biocompatibility, and environmental compliance (e.g., REACH, RoHS). Ensuring compliance mitigates market entry barriers and legal risks.
Industry and Trade Terminology for Ostomy Underwear
1. OEM (Original Equipment Manufacturer)
Refers to companies that produce ostomy underwear based on specifications provided by brand owners. Understanding OEM relationships is critical for B2B buyers seeking customized solutions, private labeling, or scalable production.
2. MOQ (Minimum Order Quantity)
The smallest quantity of products a supplier is willing to produce per order. Negotiating MOQ is vital, especially for new market entrants or smaller distributors, to balance inventory costs and supply chain flexibility.
3. RFQ (Request for Quotation)
A formal request sent by buyers to suppliers to obtain pricing, lead times, and terms for specific products. Properly prepared RFQs streamline the procurement process and enable accurate cost comparison across multiple suppliers.
4. Incoterms (International Commercial Terms)
Standardized trade terms published by the ICC that define responsibility, costs, and risks during international shipments (e.g., FOB, CIF, DDP). Clear understanding of Incoterms ensures smooth logistics, predictable costs, and legal clarity in cross-border transactions.
5. Certification and Compliance Standards
References to internationally recognized standards like ISO, ASTM, or specific regional certifications (e.g., CE, FDA). These assure buyers that products meet safety, quality, and environmental regulations necessary for market access.
6. Lead Time
The duration from order placement to product delivery. Accurate knowledge of lead times allows B2B buyers to plan inventory, coordinate supply chains, and respond swiftly to market demands, especially in regions with logistical constraints.
By mastering these technical properties and trade terms, B2B buyers from Africa, South America, the Middle East, and Europe can make informed procurement decisions, foster reliable supplier relationships, and ensure compliance with regional standards. This strategic understanding ultimately enhances product quality, reduces risks, and supports successful market expansion.
Navigating Market Dynamics, Sourcing Trends, and Sustainability in the ostomy underwear Sector
Market Overview & Key Trends
The global ostomy underwear sector is experiencing rapid growth driven by demographic shifts, technological advancements, and evolving consumer preferences. A significant driver is the increasing prevalence of colorectal cancers, IBD, and other conditions necessitating ostomy management, especially in aging populations across Europe, North America, and parts of Asia. For international B2B buyers from Africa, South America, the Middle East, and Europe, this translates into expanding market opportunities, particularly as healthcare infrastructure improves and awareness campaigns reduce stigma.
Technological innovations are shaping sourcing trends, with an emphasis on softer, breathable fabrics, discreet designs, and moisture-wicking materials that enhance comfort and skin health. Suppliers are increasingly adopting automation and digital manufacturing processes to ensure quality consistency and cost efficiency. For buyers, sourcing from regions with robust textile manufacturing capabilities—such as Turkey and South Africa—can offer a balance of quality and cost, while emerging markets like India and Southeast Asia provide cost-effective options suited for price-sensitive segments.
Market dynamics are also influenced by regulatory landscapes and reimbursement policies. In Europe, strict compliance with EU standards and a growing focus on sustainability are prompting manufacturers to incorporate recycled or bio-based materials into their products. In contrast, markets like South America and Africa often prioritize affordability and local manufacturing, leading to a demand for reliable, cost-effective solutions with simpler regulatory pathways. For B2B buyers, establishing flexible supply chains that can adapt to regional regulatory requirements and consumer preferences is crucial to capitalize on the expanding global demand.
Sustainability & Ethical Sourcing in B2B
Sustainability has become a critical factor in the sourcing and procurement of ostomy underwear, driven by environmental concerns, regulatory pressures, and shifting consumer expectations. Eco-conscious buyers are increasingly demanding products made from sustainable materials such as recycled polyester, organic cotton, or bio-based polymers that reduce carbon footprints and minimize plastic waste. Certifications like GOTS (Global Organic Textile Standard), OEKO-TEX, and Bluesign are gaining prominence as proof of ethical manufacturing and environmentally friendly practices.
Ethical sourcing extends beyond materials to encompass fair labor practices, transparent supply chains, and responsible manufacturing processes. For B2B buyers, partnering with suppliers committed to ethical standards not only mitigates reputational risks but also aligns with the growing consumer demand for socially responsible products. Traceability technologies, such as blockchain, are emerging tools to verify supply chain integrity, ensuring that raw materials are sourced sustainably and workers are treated ethically.
Furthermore, the development of biodegradable or compostable films and fabrics for ostomy underwear can significantly reduce environmental impact, especially in regions with strong waste management initiatives like Europe. Implementing green procurement policies and working with suppliers who adhere to environmental management systems like ISO 14001 can enhance brand credibility and meet stringent regulatory standards, positioning buyers as leaders in sustainable healthcare solutions.
Brief Evolution/History
The evolution of ostomy underwear and related products reflects broader advances in medical textiles and patient-centered design. Initially, ostomy products were bulky, uncomfortable, and visually conspicuous, limiting user confidence and social participation. Over the past two decades, innovations in fabric technology, adhesive systems, and product ergonomics have transformed the landscape, making ostomy underwear more discreet, comfortable, and functional.
For B2B buyers, understanding this evolution highlights opportunities for sourcing innovative materials and designs that meet both clinical and consumer needs. Early developments focused on improving barrier films and adhesives, but recent trends emphasize aesthetic appeal, breathability, and sustainability—factors that directly influence market acceptance and growth. Recognizing these shifts allows buyers to align their procurement strategies with cutting-edge innovations, ensuring competitiveness in diverse regional markets.
The ongoing integration of smart textiles and digital manufacturing further signals a future where ostomy underwear can incorporate sensors for health monitoring or adaptive features, opening new avenues for product differentiation. For international buyers, staying informed about this evolution is essential to anticipate market demands and select suppliers capable of delivering innovative, compliant, and sustainable solutions.
Related Video: International Trade Explained
Frequently Asked Questions (FAQs) for B2B Buyers of ostomy underwear
1. How can I verify the reliability and quality standards of an ostomy underwear supplier?
Ensuring supplier credibility is critical for product safety and compliance. Request ISO 13485 certification and CE or FDA approvals, depending on your target markets. Ask for comprehensive Quality Assurance (QA) documentation, including batch testing reports, biocompatibility certificates, and sterilization validations. Conduct factory audits—either virtually or onsite—to assess manufacturing practices, hygiene standards, and capacity. Additionally, review customer testimonials and request samples for independent testing. Building relationships with suppliers who transparently share their quality processes reduces risks of substandard products entering your supply chain.
2. What customization options are available for ostomy underwear to meet different market needs?
Suppliers typically offer a range of customization options to cater to regional preferences and regulatory requirements. Common customizations include size ranges, color options, packaging designs, and branding. Advanced features such as moisture-wicking fabrics, discreet designs, or specific closure mechanisms can be tailored. For markets emphasizing comfort and discretion, request softer, breathable materials and low-profile designs. Ensure your supplier can accommodate small to large order quantities for prototypes and bulk production. Clarify lead times for customized products, especially if they involve specialized materials or branding, to align with your distribution plans.
3. What are typical minimum order quantities (MOQs), lead times, and payment terms for sourcing ostomy underwear internationally?
Most manufacturers set MOQs based on production costs, often ranging from 1,000 to 10,000 units for standard products. Lead times vary from 4 to 12 weeks depending on complexity, customization, and current production schedules. Payment terms commonly include 30% upfront, with the balance payable before shipment or upon delivery. Some suppliers may offer flexible terms for repeat buyers or large orders, including letter of credit or bank transfer options. Negotiating MOQs and lead times early helps align production with your inventory needs and minimizes cash flow strain, especially in markets with longer logistics cycles.
4. What certifications and documentation should I request to ensure compliance with international standards?
Request relevant certifications such as ISO 13485 for medical devices, CE marking for European markets, FDA clearance for the US, and country-specific approvals like Japan’s PMDA. Additionally, ask for biocompatibility reports, sterilization certificates, and batch testing results. For logistics, ensure suppliers provide commercial invoices, packing lists, and certificates of origin. Having these documents not only confirms compliance but also facilitates customs clearance and reduces delays. Verifying supplier adherence to international quality standards mitigates legal and safety risks, especially when distributing in highly regulated markets.

Illustrative Image (Source: Google Search)
5. How should I handle logistics and shipping when importing ostomy underwear into different regions?
Coordinate with suppliers experienced in international freight, who can advise on the most cost-effective and reliable shipping methods—air freight for urgent needs or sea freight for bulk orders. Clarify Incoterms (e.g., FOB, CIF) to understand responsibilities and costs. Ensure the supplier provides comprehensive shipping documentation for smooth customs clearance, including certificates of origin and import permits if required. Consider partnering with freight forwarders specializing in healthcare products to handle complex logistics and ensure timely delivery. Also, account for regional customs regulations and import duties, which vary across Africa, South America, the Middle East, and Europe.
6. What are common dispute resolution mechanisms in international B2B transactions for medical products?
Establish clear contractual terms upfront, including dispute resolution clauses. Preferred mechanisms include arbitration under recognized rules such as ICC or LCIA, which offer neutrality and enforceability across borders. Alternatively, specify jurisdiction (e.g., courts in the supplier’s country or your own) and applicable law. Ensure contracts specify quality standards, delivery timelines, and penalties for non-compliance. Maintaining transparent communication and documentation helps resolve issues swiftly. Working with legal advisors familiar with international trade law can prevent costly misunderstandings and provide guidance on effective dispute management.
7. How can I assess the supplier’s ability to meet future demand and scale production?
Evaluate their production capacity, flexibility, and supply chain resilience through site visits or detailed questionnaires. Ask about their manufacturing lead times, inventory management systems, and contingency plans for raw material shortages. Reliable suppliers often have multiple production lines and diversified sourcing for key components. Request references from existing clients in similar markets to gauge their scalability. Building a long-term partnership with suppliers capable of scaling ensures continuous supply, especially as demand increases in emerging markets or during product launches.

Illustrative Image (Source: Google Search)
8. What are best practices for establishing long-term relationships with international suppliers of ostomy underwear?
Start with clear, detailed contracts specifying quality standards, lead times, payment terms, and communication channels. Maintain regular contact through video calls and site visits when possible, fostering transparency and trust. Provide feedback on product performance and collaborate on innovations tailored to specific regional needs. Establish mutually agreed key performance indicators (KPIs) to monitor delivery, quality, and responsiveness. Diversify your supplier base to reduce risks and encourage competitive pricing. Building partnerships rooted in transparency, reliability, and shared growth objectives leads to sustainable, long-term success in the global ostomy care market.
Strategic Sourcing Conclusion and Outlook for ostomy underwear
Strategic Sourcing Outlook and Key Takeaways
Effective strategic sourcing in the ostomy underwear sector hinges on understanding regional market dynamics, regulatory landscapes, and evolving patient needs. For international buyers from Africa, South America, the Middle East, and Europe, aligning supply chain strategies with regional demand drivers—such as aging populations, rising disease prevalence, and sustainability trends—is crucial. Prioritizing suppliers with robust compliance capabilities and innovative product offerings ensures access to high-quality, compliant products that meet local standards and patient expectations.
Building resilient, flexible supply chains offers a competitive advantage amid regulatory complexities and fluctuating demand patterns. Emphasizing partnerships with manufacturers committed to technological advancements—such as breathable, discreet, and eco-friendly materials—can differentiate your portfolio. As markets continue to evolve, proactive sourcing strategies that incorporate regional regulatory insights, reimbursement policies, and sustainability requirements will be vital.
Looking ahead, international B2B buyers should focus on establishing long-term supplier relationships, leveraging local market intelligence, and investing in innovation. This approach will position your organization to capitalize on emerging opportunities, meet increasing demand, and deliver superior value to patients worldwide. Embrace strategic sourcing as a key driver of growth and resilience in the dynamic ostomy underwear landscape.